In this notes or relationship of Haematoxylin and Haematin, you'll get an Introduction to Haematoxylin along with Haematin - an overview, Haematoxylin - an overview. Also, it will explain haematoxylin colour, uses of haematoxylin and hematoxylin stain. What happens on the rapid conversion of haematoxylin into haematin.
Relationship between Haematoxylin and Haematin
-
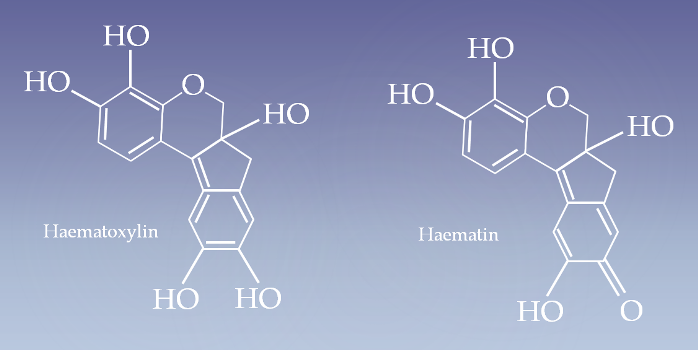
Haematoxylin is extracted from the heartwood of logwood tree. When oxidized it forms Haematin, a compound that forms strongly coloured complexes with certain metal ions, the most notable ones being Fe(III) and Al(III) salts.
-
Haematin exhibits indicator like properties, being blue and less soluble in aqueous alkaline conditions, and red and more soluble in alcoholic acidic conditions. Dissolved haematin slowly reacts with atmospheric oxygen, yielding products that are not useful.
-
Haematoxylin is relatively soluble in aqueous solution.
-
Haematoxylin and eosin stain is one of the most commonly used stains in histology. It is a permanent stain as opposed to temporary stains. Another common stain is phosphotungstic acid
-
In Acidic solutions complexes of haematin with metals(Usually aluminium or iron, but also chromium, Zirconium and several others) are used as biological stains, Aluminium-haematin(haemalum) is the “routine” stain for cell nuclei in sections of human and other animal tissues.
-
The three main alum haematoxylin solutions employed are Ehrlich’s haematoxylin, Harris’s haematoxylin and Mayer’s haematoxylin. The name haemalum is preferable to “haematoxylin” for these solutions because haematin, a product of oxidation of haematoxylin, is a3t compound that combines with aluminium ions to form the active dye metal complex.
- 116 reads